ONKOSIGHT ADVANCEDTM TUMOR MUTATION BURDEN (TMB) AND MICROSATELLITE INSTABILITY (MSI)
OnkoSight AdvancedTM Next-generation Sequencing accurately measures key immunotherapy biomarkers; Tumor Mutation Burden (TMB) and Microsatellite Instability (MSI).
Tumor Mutation Burden (TMB)
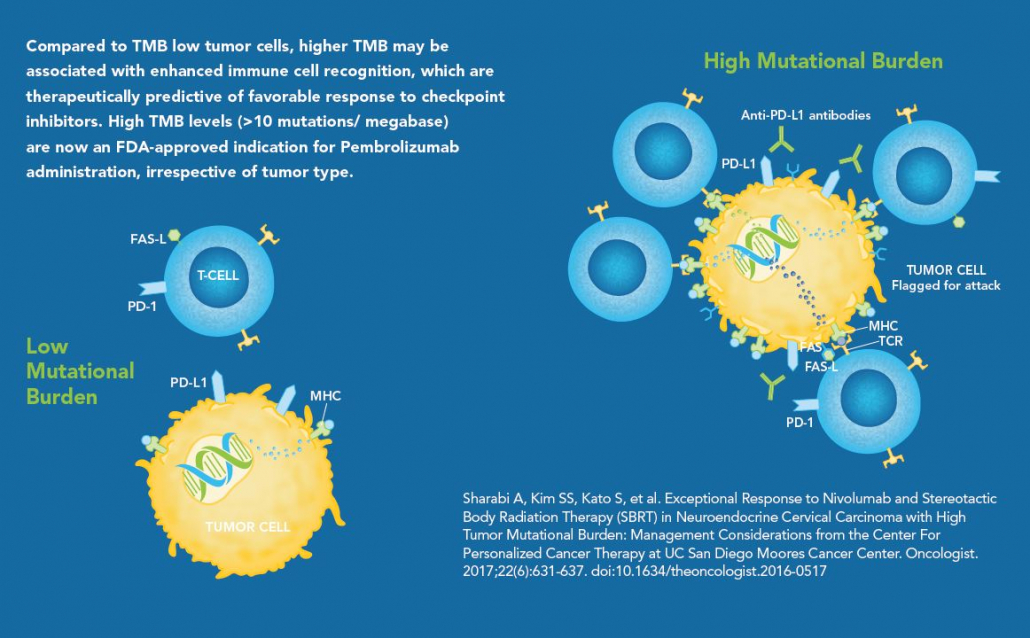
The significance of Tumor Mutation Burden (TMB) as a critical molecular biomarker in clinical oncology is being increasingly recognized.
- High TMB levels are known to be especially prevalent among non-small cell lung cancer (NSCLC), melanoma, squamous cell carcinoma of the head and neck, and urothelial carcinoma, among others.1-6
- Presence of > 10 mutations/megabase has been reported to be therapeutically predictive of favorable clinical outcomes and responsiveness to immune checkpoint inhibitors.7
Microsatellite Instability (MSI)
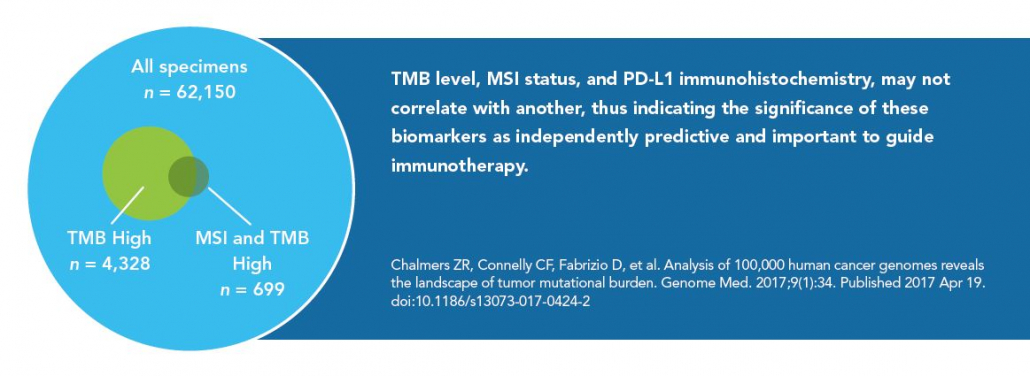
Microsatellite Instability (MSI) status is among the first truly histology agnostic biomarkers relevant to guide immunotherapy, critical to precision medicine approaches in clinical oncology.
- In comparison to traditional PCR, which typically interrogates only 5 microsatellite loci, OnkoSight AdvancedTM NGS targets a total of 130 homopolymer sites. An accumulation of data suggests that MSI calling by PCR may demonstrate inferior accuracy and technical sensitivity when only a small number of loci are examined.8
A comprehensive testing strategy for both TMB and tumor-only MSI is now recognized as an emerging standard of care for contemporary genomic profiling of advanced solid organ malignancies. Conjunctional NGS MSI testing eliminates the requirement for iterative testing approaches and mitigates risk of tissue depletion in scant specimens. 9-10
References:
- Stenzinger A, Allen JD, Maas J et al., Tumor Mutation Burden Standardization initiatives: Recommendations for consistenttumor mutational burden assessment in clinical samples to guide immunotherapy treatment decision Gene Chromosomes Cancer 2019 Aug:58(8):578-588 doi:10.1002/gcc.22733 Epub 2019 Mar 7
- Buttner et al (2019) ESMO Open. Jan 24; 4(1)
- Hellman et al (2018) New England Journal of Medicine 378 (22): 2093-2104
- Ready, K et al (2019). Journal of Clinical Oncology 37 (12): 992-1000
- Rizvi et al. (2015). Science 348 (6230): 124-128
- Journal for Immunotherapy for Cancer 2018, 6 (Suppl 2):O48
- N Engl J Med 2018; 378:2093-2104
- Macus L, Lemery S et al, FDA approval Summary: Pembrolizumab for the treatment of Microsatellite Instability-High Solid Tumors. PubMed ID: 30787022
- Vanderwalde A, Spetzler D et al Microsatellite Instability Status Determined by Next-Generation Sequencing and Compared with PD-L1 and Tumor Mutation Burden in 11,348 Patients. PubMed ID: 29436178
- Kautto E, Bonneville R et al Performance Evaluation for Rapid Detection of Pan-Cancer Microsatellite Instability with MANTIS. PubMed ID: 27980218