OnkoSight AdvancedTM Reanalysis
Test Code: TM56-4
OnkoSight AdvancedTM, a cutting-edge next-generation sequencing (NGS) assay from GenPath®, allows reanalysis of previously established sequencing data using extended gene panels, up-to-date guidelines, and new variant-disease associations.
Focusing on patients
At GenPath we take a guideline-driven approach in the design of our panels but we also understand that in certain circumstances healthcare providers need to look beyond guidelines for therapy options. Reanalysis solves that very problem and enables clinicians to initially use a smaller, targeted panel to treat their patients and then, if required, have over 500 genes reanalyzed without the need for re-sequencing.

How does it work?
Previously reported genomic data centered around core gene variants specifically associated with U.S Food and Drug Administration and National Comprehensive Cancer Network® guidelines formulated for morphologic tumor subtypes are biocomputationally broadened to include sequencing data on >500 whole genes and low-pass whole genome structural variation supported with up-to-date interpretation and disease annotation.
OnkoSight Advanced Reanalysis will not require new sample submission or sequencing.
The test is offered on a self-pay basis only. GenPath requires submission of a signed Notice of Patient Financial Responsibility Form when ordering the test.
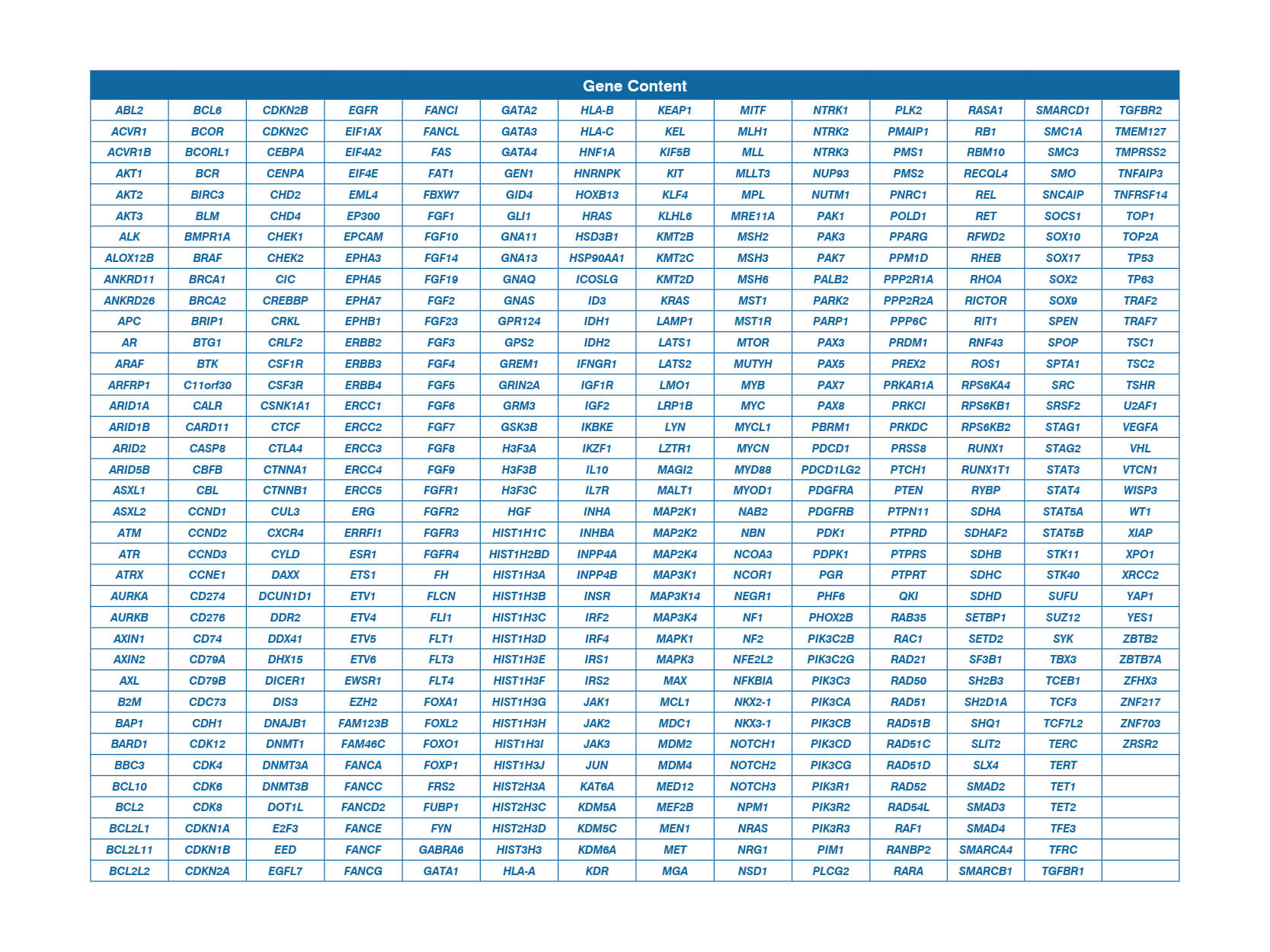
OnkoSight Advanced 523 Gene Panel Component
Healthcare providers should only order panels if each gene or test in the panel is medically necessary.